Expert Consulting in Medical Devices MDR & IVDR Regulations
Internal Audit
ISO13485 / MDR / IVDR
€1,500.00*
Specialized internal audit service for companies in the medical devices sector and in vitro diagnostic medical devices sector.
Supplier Audits
€1,500.00*
Make your life easier—let us handle your supplier audits. We conduct comprehensive evaluations of your critical suppliers using methodology based on ISO 19011 , ISO 13485:2016 and applicable regulatory requirements.
Consulting for MD and in-vitro devices per hour
€75.00*
Navigating the regulatory journey together. We guide you through compliance with MDR (EU) 2017/745, IVDR (EU) 2017/746, FDA 21 CFR 820, and ISO 13485 to bring your medical device to market.
Medical Device regulatory Training
from €500.00* per 10 persons
Formación técnica especializada para tu equipo. Capacitación práctica impartida por expertos regulatorios con experiencia en auditorías e implementación.
4 hours package - Choose your training course.
Ongoing regulatory support service
from €500.00* per month
Navigating the regulatory journey together.
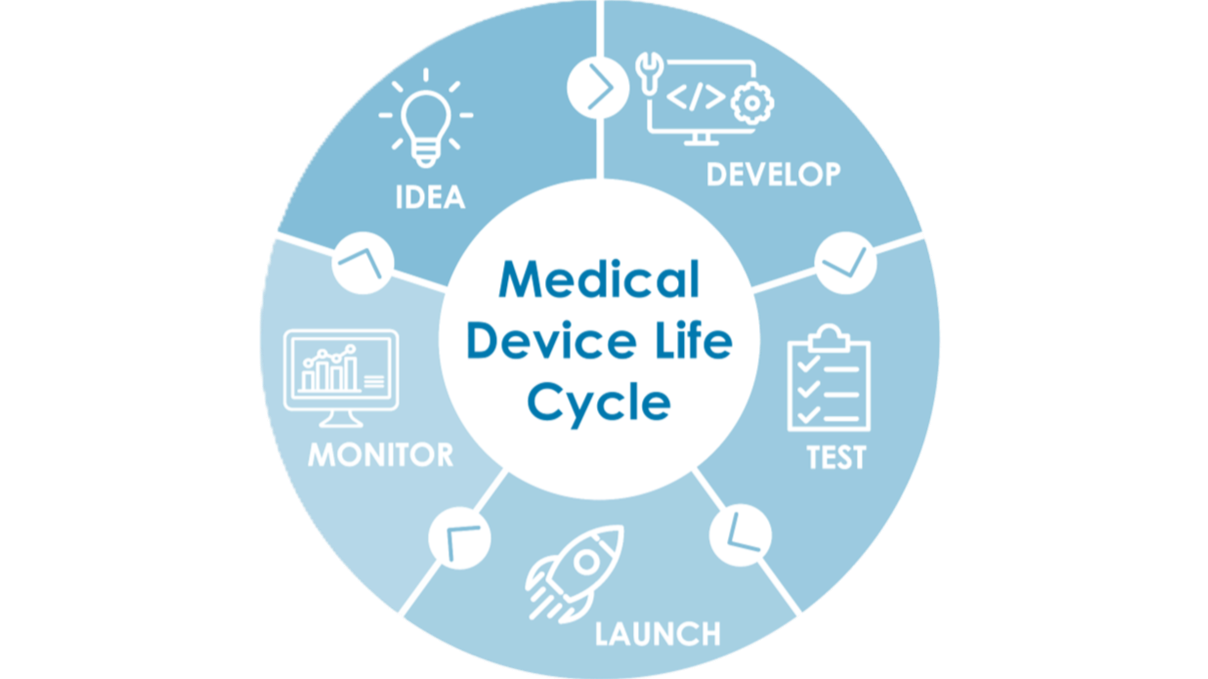
Flexible technical-regulatory support tailored to your needs. Your strategic partner throughout the entire product lifecycle: from initial concept to post-market surveillance.
May I help you?
I will contact you as soon as possible.